Magnesium Die Castings offer significant advantages in weight reduction and performance across various industries. The market for these parts shows robust growth, with projections indicating an increase from USD 3.3 Billion in 2024 to USD 5.5 Billion by 2033. This growth highlights their increasing adoption.
However, magnesium’s inherent electrochemical reactivity makes these parts susceptible to corrosion, posing a critical challenge for their durability. Fortunately, effective strategies exist to significantly enhance and maintain the Corrosion Resistance of these components.
Key Takeaways
- Magnesium die castings are popular for their light weight, but they can rust easily.
- Keep impurities like iron, nickel, and copper very low in magnesium alloys to stop rust.
- Adding aluminum to magnesium alloys helps them resist rust better by forming a protective layer.
- Special surface treatments like PEO/MAO create a hard, ceramic-like coating that protects magnesium.
- Using paints or powder coatings forms a barrier to keep moisture and rust away from magnesium parts.
- Combine different coatings, like a base layer and a topcoat, for stronger protection against rust.
- Design parts carefully to avoid small gaps and moisture traps where rust can start.
- Regular checks and cleaning help find and fix rust early, making magnesium parts last longer.
Understanding Magnesium’s Susceptibility to Corrosion
Fundamental Principles of Magnesium Corrosion
Electrochemical Reactivity of Magnesium
Magnesium exhibits high electrochemical reactivity. It readily loses two valence electrons, a characteristic of Group II elements. This low ionization energy means little energy is required for magnesium to lose these two electrons. This makes it prone to forming oxides, hydroxides, or sulfides, initiating corrosion. Corrosion is an electrochemical redox (reduction/oxidation) reaction where a metal loses electrons.
Anodic Behavior in Aqueous Environments
In aqueous environments, magnesium typically acts as the anode. It loses electrons to oxygen, forming magnesium oxide (2Mg + O2 → 2MgO). This process signifies its anodic behavior, where the metal itself oxidizes and degrades. This inherent tendency makes magnesium susceptible to various forms of corrosion when exposed to moisture or electrolytes.
Key Factors Influencing Magnesium Corrosion
Role of Metallic Impurities (Fe, Ni, Cu)
Metallic impurities significantly accelerate magnesium corrosion. Elements like iron (Fe), nickel (Ni), and copper (Cu) increase corrosion rates. Even when the concentration of these impurities is less than 0.2%, they negatively affect magnesium alloys. Iron increases corrosion by forming an electric couple with magnesium. Nickel, with its very low solid solubility, accelerates corrosion even in small amounts. Copper forms intermetallic compounds with magnesium, distributing around grain boundaries and effectively accelerating the corrosion rate. To enhance Corrosion Resistance, manufacturers strictly control impurity content during raw material processing and smelting. Allowable impurity limits for magnesium alloys in NaCl solution are suggested as: Fe ≤ 0.032 × Mn%, Ni ≤ 0.001%, and Cu ≤ 0.04%. Within these ranges, corrosion resistance remains largely unaffected.
Impact of Environmental Conditions (Humidity, Chlorides)
Environmental conditions, particularly humidity and chlorides, significantly impact magnesium corrosion. Cui et al. found the corrosion rate of AZ31 was 17.66 μm/a in Xisha, an environment with high temperature, high humidity, and high salt spray. Yu et al. observed a significant effect of relative humidity (RH) on pure Mg, AM60, and AZ91D alloys in a real marine atmosphere. LeBozec et al.’s indoor simulations showed increasing relative humidity from 75% to 95% increased the corrosion rate. This occurs because a thicker electrolyte layer forms at higher RH, leading to less protective corrosion product layers. Liao et al. found higher corrosion rates in coastal environments like Shimizu City due to sea salt influence, with chloride deposition frequently above 100 mg/m²·d. Laboratory exposures also demonstrated a linear increase in the corrosion rate of AZ91 and AM50 with chloride deposition.
Galvanic Corrosion Mechanisms with Dissimilar Metals
Galvanic corrosion occurs when magnesium contacts a more noble metal in the presence of an electrolyte. Magnesium, being more active, acts as the anode and corrodes preferentially. This mechanism is particularly problematic when magnesium parts are joined with materials like steel or copper without proper isolation.
Common Forms of Corrosion in Magnesium Die Castings
Pitting Corrosion Characteristics
Pitting corrosion is a localized form of attack. It creates small holes or pits on the magnesium surface. This type of corrosion often initiates at defects or impurity sites, leading to concentrated damage.
Uniform Corrosion Patterns
Uniform corrosion involves a general attack across the entire exposed surface of the magnesium part. This results in a relatively even thinning of the material. While less localized, it can still lead to significant material loss over time.
Stress Corrosion Cracking (SCC) Risks
Stress corrosion cracking (SCC) is a dangerous form of corrosion. It occurs when tensile stress and a corrosive environment act simultaneously on the magnesium part. SCC can lead to sudden and unexpected failure, even at stresses below the material’s yield strength.
Filiform Corrosion Appearance
Filiform corrosion appears as thread-like filaments growing under a coating. It typically starts at small defects in the coating and spreads outwards. This form of corrosion can compromise the integrity and appearance of coated magnesium parts.
Enhancing Corrosion Resistance Through Alloy Selection
High-Purity Magnesium Alloys for Improved Corrosion Resistance
Importance of Ultra-Low Impurity Levels
Metallic impurities significantly influence the corrosion behavior of magnesium alloys. Elements such as iron (Fe), nickel (Ni), and copper (Cu) act as cathodic sites. These sites accelerate the electrochemical reaction, leading to increased corrosion rates. Manufacturers strictly control impurity levels to mitigate this effect. Ultra-low impurity levels minimize the formation of these detrimental cathodic sites. This approach ensures a more uniform and stable surface, which resists corrosive attack more effectively.
Specific High-Purity Alloy Compositions
High-purity magnesium alloys specifically target applications demanding superior corrosion performance. These alloys achieve their enhanced properties through stringent control over their chemical composition. They maintain impurity levels well below the thresholds found in commercial-grade magnesium. Examples include specialized grades of pure magnesium (HP-Mg) and certain high-purity variants of common alloys like AZ91. These compositions prioritize the reduction of cathodic impurities to fractions of a percent.
Corrosion Performance Benefits of High Purity
High-purity magnesium alloys offer substantial improvements in corrosion performance. Their reduced impurity content directly translates into lower corrosion rates. This makes them suitable for more demanding environments. The table below illustrates the significant difference in corrosion rates between high-purity and commercial-purity magnesium.
| Material Type | Corrosion Rate (mm y⁻¹) | Equivalent Corrosion Rate (mg cm⁻² day⁻¹) |
|---|---|---|
| High-Purity Magnesium (HP-Mg, ≥ 99.99 wt. %) | 0.3-0.5 | N/A |
| Commercial Pure Magnesium (CP-Mg, ≤ 99.95 wt. %) | 6.26 | 3.00 |
This data clearly shows high-purity magnesium exhibits a significantly lower corrosion rate, indicating its superior resistance to degradation.
Role of Aluminum in Magnesium Alloy Corrosion Resistance
AZ91D: A Benchmark for Corrosion Resistance
AZ91D stands as a benchmark alloy for magnesium die castings due to its excellent combination of mechanical properties and good corrosion resistance. This alloy contains approximately 9% aluminum and 1% zinc. The aluminum content plays a crucial role in its enhanced corrosion performance. AZ91D finds widespread use in automotive and electronic components where a balance of strength, castability, and corrosion protection is essential.
AM Series Alloys (AM60B, AM50A) and Their Properties
AM series alloys, such as AM60B and AM50A, represent another class of magnesium-aluminum alloys. They offer specific advantages in terms of ductility and impact resistance. These alloys typically contain lower aluminum content than AZ91D.
- AZ91D’s Corrosion Mechanism:
- Alloy AZ91 exhibits significantly less localized corrosion in humid air compared to alloy AM50 and commercial pure magnesium.
- Increasing the aluminum content in Mg–Al alloys leads to a significant increase in the protective properties of the surface film.
- The improved corrosion resistance is attributed to the presence of a continuous skeletal barrier of alumina (Al2O3) in the film.
- Exposure of alloy AM50 to NaCl (aq) solution results in the formation of a thin protective Al-rich layer at the metal-surface film interface, particularly in regions with high aluminum content.
- Analysis by XPS demonstrates the presence of small amounts of Al3+ in the surface film on alloys AM50 and AZ91, with the concentration of aluminum increasing towards the bottom of the film.
- The slow corrosion of alloy AZ91 is suggested to be due to its relatively high aluminum content, and the presence of aluminum in the surface film enhances its ability to protect against corrosion.
- Aluminum is suggested to accumulate between MgO grains in the form of Al2O3 or spinel (MgAl2O4), providing corrosion protection.
Some researchers suggest that the aluminum enrichment at the alloy/oxide interface has a ‘metallic character’. Other researchers believe that the enrichment is due to the formation of oxidized aluminum (Al3+).
- AM60B and AM50A Characteristics:
- AM60B Composition: 5.5 to 6.5% Al, 0.25% Mn min., 0.10% Si max., 0.22% Zn max., 0.005% Fe max., 0.010% Cu max., 0.002% Ni max., 0.003% max. other (total), Balance Mg.
- AM60B Performance: This alloy offers high toughness and impact resistance (elongation 8-10%, tensile strength ~180 MPa, yield strength ~85 MPa), making it suitable for dynamic loads. It provides excellent corrosion resistance, about 30% higher salt spray resistance than AZ91D (150-250 hours). This superior performance results from manganese forming high-melting-point compounds that reduce intergranular corrosion.
- AM50A Composition: 4.5-5.3% Al, ≥0.28% Mn, balance Mg.
- AM50A Performance: This alloy shows improved plasticity (elongation up to 12%) due to its lower aluminum content, though its strength is slightly reduced (tensile strength ~160 MPa). It also exhibits excellent weldability and corrosion resistance.
Corrosion resistance rapidly decreases if the manganese content is less than 0.25% or the iron content exceeds 0.005%, or if the Fe-Mn ratio exceeds 0.010. Corrosion resistance also decreases with increasing iron, copper, or nickel content.
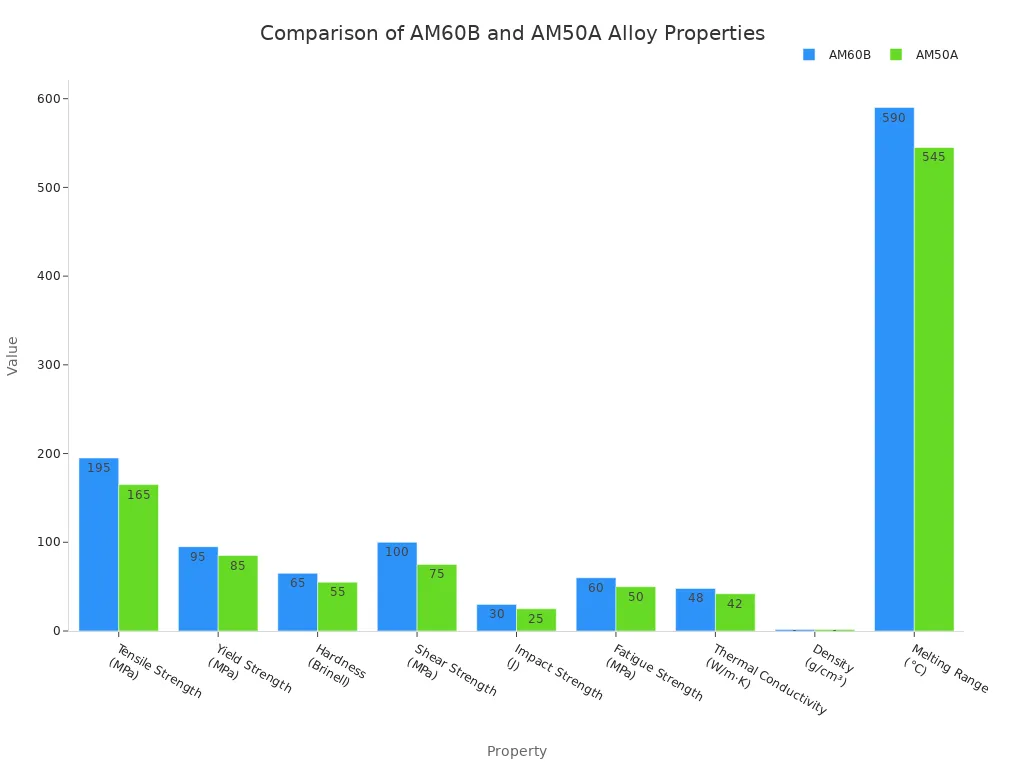
The table below provides a comparison of key mechanical properties for AM60B and AM50A alloys.
| Property | AM60B | AM50A |
|---|---|---|
| Tensile Strength (MPa) | 195 | 165 |
| Yield Strength (MPa) | 95 | 85 |
| Hardness (Brinell) | 65 | 55 |
| Shear Strength (MPa) | 100 | 75 |
| Impact Strength (J) | 30 | 25 |
| Fatigue Strength (MPa) | 60 | 50 |
| Thermal Conductivity (W/m·K) | 48 | 42 |
| Density (g/cm³) | 1.81 | 1.81 |
| Melting Range (°C) | 540-640 | 510-580 |
AM60B is recognized for its excellent castability, impact resistance, and overall strength. This makes it suitable for components in power tools and lighting solutions where precision and durability are crucial. AM50A offers a balance of strength and flexibility. This makes it a prime choice for medical devices due to its biocompatibility and lightweight nature, which are important for intricate and reliable medical instruments.
Rare Earth (RE) Containing Magnesium Alloys
Mechanisms of RE Elements in Corrosion Inhibition
Rare earth (RE) elements, such as Cerium (Ce) and Lanthanum (La), inhibit corrosion in magnesium alloys through several mechanisms. One key mechanism involves the stabilization of corrosion products. Additionally, adding RE elements to an AM60 alloy improves its corrosion resistance by eliminating micro-galvanic couplings. The inclusion of Yttrium (Y) and Lanthanum (La) in TX31 alloys enhances corrosion film resistance and reduces corrosion rates. Furthermore, the formation of Y2O3 and Y(OH)3 creates a protective barrier against corrosion, as seen in Mg-3.21Y-3.15 La alloys. While La(OH)3 formation can lead to crater structures, the overall effect of RE additions is to improve corrosion resistance.
Enhanced Corrosion Resistance of AE42 and AJ62
AE42 and AJ62 are prominent examples of RE-containing magnesium alloys. These alloys incorporate elements like cerium and lanthanum. The presence of these rare earth elements refines the grain structure and modifies the intermetallic phases. This leads to a more uniform corrosion attack and reduces localized pitting. AE42, for instance, offers improved creep resistance at elevated temperatures, making it suitable for powertrain applications. AJ62 provides a good balance of mechanical properties and enhanced corrosion performance, particularly in high-temperature environments.
Applications Benefiting from RE Alloys
RE-containing magnesium alloys find applications in industries requiring high performance under challenging conditions. The automotive industry utilizes them for engine blocks, transmission casings, and other components exposed to elevated temperatures. Aerospace applications also benefit from their lightweight and improved high-temperature properties. Their enhanced corrosion resistance makes them valuable in environments where conventional magnesium alloys might quickly degrade.
Specialized Magnesium Alloys for Specific Environments
Alloys for High-Temperature Corrosion Resistance
Magnesium alloys often face challenges in high-temperature applications. Elevated temperatures can accelerate oxidation and creep, compromising material integrity. Specialized magnesium alloys address these issues. These alloys incorporate elements like yttrium (Y), scandium (Sc), and other rare earth (RE) elements. Yttrium, for example, significantly improves the high-temperature strength and creep resistance of magnesium alloys. It forms stable intermetallic phases that resist deformation at elevated temperatures. Scandium also enhances strength and refines grain structure, contributing to better performance under heat.
The WE series alloys (e.g., WE43, WE54) exemplify high-temperature magnesium alloys. They contain yttrium and other rare earth elements. These alloys maintain their mechanical properties and structural stability even at temperatures exceeding 200°C. This makes them suitable for demanding applications in the automotive and aerospace industries. For instance, manufacturers use them in engine components, gearbox casings, and aircraft parts where heat exposure is constant. These specialized compositions ensure the parts retain their functionality and structural integrity in hot operating conditions.
Alloys for Marine and Chloride-Rich Environments
Chloride-rich environments, such as marine settings, pose a significant threat to many metals, including magnesium. The presence of chloride ions aggressively attacks the protective oxide layer on magnesium, leading to rapid degradation. Developing alloys for these conditions requires careful material design. Researchers focus on creating alloys that form more stable and protective passive films in the presence of chlorides.
High-purity magnesium alloys, with their extremely low levels of iron, nickel, and copper, offer improved Corrosion Resistancein chloride environments. These impurities act as cathodic sites, accelerating galvanic corrosion. By minimizing them, the overall corrosion rate decreases. Furthermore, certain rare-earth-containing alloys show promise in marine applications. These elements can modify the corrosion product layer, making it more adherent and less permeable to corrosive agents. For example, alloys with specific additions of cerium or lanthanum can form denser, more protective films. These films effectively shield the underlying magnesium from chloride attack. Such alloys find use in marine equiPment, offshore platforms, and other applications exposed to saltwater.
Surface Treatment Technologies for Superior Corrosion Resistance

Conversion Coatings for Initial Corrosion Protection
Chromate Conversion Coatings: Benefits and Limitations
Chromate conversion coatings have historically provided excellent initial protection for magnesium die castings. These coatings offer exceptional corrosion protection by forming a durable surface barrier. They also enhance paint adhesion, creating a porous structure for better bonding. Furthermore, they provide some electrical conductivity, which is useful for electronic applications. These coatings improve the overall appearance with a uniform, smooth finish and are available in various colors. They significantly extend the lifespan of metal parts due to their durability and longevity.
Despite these benefits, chromate conversion coatings, particularly those involving toxic hexavalent chromium (Cr6) ions, face significant limitations. Environmental concerns and legislative restrictions, such as EU directives 2002/95/EC and 2002/96/EC, have led to their phasing out. The excellent performance of hexavalent chromate conversion coatings, including their self-repair ability, makes finding viable alternatives challenging. This is especially true for industries like aeronautics, which are major consumers of chromates.
Phosphate Conversion Coatings: Application and Performance
Phosphate conversion coatings offer another method for protecting magnesium surfaces. Manufacturers apply these coatings using various phosphate types, including magnesium phosphate, calcium phosphate, manganese phosphate, and zinc phosphate. Pretreatment techniques like laser surface textured, laser shock peening, and permanganate-phosphate solutions can optimize their application. Factors such as pH value and preparation temperature significantly influence coating formation.
Magnesium phosphate conversion coatings (MPCC) on AZ31 Mg alloy notably enhance corrosion resistance. Analysis shows the coating consists of magnesium phosphate and magnesium hydroxide/oxide compounds. The corrosion current of the MPCC was reduced to approximately 3% of the uncoated surface. The time until deterioration during immersion in a 0.5 mol/L NaCl solution improved from about 10 minutes to approximately 24 hours.
Fluoride Conversion Coatings: Mechanism and Use
Fluoride conversion coatings provide a protective layer on magnesium surfaces by reacting with the metal to form insoluble magnesium fluoride. This layer acts as a barrier, isolating the magnesium from corrosive environments. Manufacturers typically apply these coatings through immersion in fluoride-containing solutions. The resulting film offers good adhesion and can serve as a base for subsequent organic coatings. Fluoride coatings are particularly effective in environments where mild chemical resistance is required. They contribute to the overall durability of magnesium components.
Environmentally Friendly Alternatives to Chromates
The demand for environmentally friendly alternatives to chromate coatings has led to the development of several effective solutions. Plasma Electrolytic Oxidation (PEO) is one such alternative. It forms hard, dense, and wear-resistant coatings on lightweight metals like magnesium. This process uses plasma discharges to transform the metallic surface, creating an adhesive oxide layer. PEO coatings exhibit higher hardness, chemical passivity, and an advantageous, irregular pore structure. This results in high strain tolerance and stronger adhesion compared to anodized coatings. The PEO process is environmentally friendly due to its use of benign electrolytes, which are free from acids, ammonia, heavy metals, and chromium. It also produces non-toxic byproducts.
Other chromate-free conversion coatings, such as SurTec 650V and Iridite NCP, also offer excellent performance. These trivalent chromium-based coatings are applied via immersion or spray. They provide superior corrosion protection and paint adhesion without using toxic hexavalent chromium. Both are environmentally friendly, REACH and RoHS compliant, and operate at room temperature with short processing times. They are compatible with various magnesium alloys and find use in aerospace, automotive, and electronics industries.
| Feature | SurTec 650V | Iridite NCP |
|---|---|---|
| Type | Chromate-free conversion coating | Chromate-free conversion coating |
| Composition | Based on trivalent chromium | Based on trivalent chromium |
| Application | Immersion or spray application | Immersion or spray application |
| Corrosion Resistance | Excellent, comparable to hexavalent chromium coatings | Excellent, comparable to hexavalent chromium coatings |
| Adhesion | Provides an excellent base for subsequent painting or bonding | Provides an excellent base for subsequent painting or bonding |
| Environmental Impact | Environmentally friendly, REACH and RoHS compliant | Environmentally friendly, REACH and RoHS compliant |
| Key Benefit | Offers superior corrosion protection and paint adhesion without the use of toxic hexavalent chromium | Offers superior corrosion protection and paint adhesion without the use of toxic hexavalent chromium |
| Specific Use Cases | Often used in aerospace, automotive, and electronics industries for magnesium alloys | Widely used in various industries for corrosion protection and paint adhesion on magnesium alloys |
Anodizing Processes for Robust Corrosion Resistance
Plasma Electrolytic Oxidation (PEO) / Micro-Arc Oxidation (MAO)
Plasma Electrolytic Oxidation (PEO), also known as Micro-Arc Oxidation (MAO), represents an advanced anodizing process. It creates highly protective ceramic-like coatings on magnesium alloys. This electrochemical surface treatment involves generating plasma discharges on the metal surface in an electrolyte. These discharges transform the surface into a dense, hard oxide layer.
Characteristics of PEO/MAO Layers
PEO/MAO layers are characterized by their ceramic nature. They are typically dense, hard, and highly adherent to the magnesium substrate. These coatings often exhibit a porous outer layer and a denser inner layer, providing a robust barrier against environmental attack. The ceramic composition contributes significantly to their protective qualities.
Enhanced Hardness and Corrosion Resistance
MAO coatings significantly enhance the wear resistance and corrosion performance of magnesium. MAO creates a ceramic layer that is both lightweight and extremely durable. Magnesium substrates can achieve extremely hard ceramic surfaces through MAO. Materials with stable oxide layers provide superior long-term protection. This makes PEO/MAO-treated parts suitable for demanding applications requiring high durability.
Process Parameters and Coating Properties
Several process parameters influence the properties of PEO/MAO coatings. Higher voltage and current density increase coating growth rates. However, they can also lead to larger pores and cracks due to excessive plasma intensity. Pulse frequency and duty cycle regulate the timing and intensity of individual discharges. These parameters influence oxide growth and defect nucleation. Electrolyte chemistry is crucial for controlling discharge mechanisms, coating growth kinetics, and the formation of oxide phases. It also enables the incorporation of elements like Si, P, and Al, which enhance mechanical and protective properties.
Specific electrolyte chemistries include:
- Alkaline Silicate Solutions (e.g., Na2SiO3, K2SiO3): These promote silicon incorporation and the formation of hard silicate phases.
- Phosphate-based Systems (e.g., Na3PO4, Na2HPO4): These enhance corrosion resistance by introducing Mg–P–O compounds.
- Aluminate-based Systems (e.g., NaAlO2): These improve wear resistance and microhardness.
- Blended Systems: Manufacturers use these to integrate hardness, adhesion, and corrosion resistance by combining different electrolyte components.
A specific PEO process for magnesium involved a square-voltage waveform. It was carried out for 12 minutes in three steps:
- Step I (70 s): Linear voltage ramping from 5 V to 195 V.
- Step II (450 s): Voltage linearly increased to 265 V, with a superimposed pulsed voltage signal growing from 0 V to 46 V.
- Step III (200 s): Terminal voltage levels were constant, with current intensity gradually fading, indicating self-limiting coating growth.
Other parameters for this specific process included:
- Duty Cycle: 30%
- Electrolyte Temperature: Maintained at 303 ± 5 K with continuous stirring.
- Electrolyte Composition: A solution of sodium metasilicate (Na2SiO3) at 15 g/L, sodium hydroxide (NaOH) at 2.5 g/L, and disodium hydrogen phosphate (Na2HPO4) at 1 g/L.
- Independent Parameters Varied: Peak current densities of 10, 12, and 14 A/dm² and pulse frequencies of 1000, 1500, and 2000 Hz.
Organic Coatings as Barrier Protection for Corrosion Resistance
Types of Paints and Lacquers for Magnesium
Organic coatings, such as paints and lacquers, provide an effective barrier against corrosive agents for magnesium die castings. These coatings physically separate the metal surface from the environment. Common types include epoxy, polyurethane, and acrylic-based formulations. Epoxy paints offer excellent adhesion and chemical resistance. Polyurethane coatings provide good abrasion resistance and flexibility. Acrylic lacquers offer good UV stability and aesthetic appeal. The selection depends on the specific application and environmental exposure.
Powder Coatings: Application and Durability
Powder coatings offer a durable and environmentally friendly alternative to liquid paints. Manufacturers apply them as a dry powder, then cure them under heat to form a hard, protective layer. This process eliminates the need for solvents, reducing volatile organic compound (VOC) emissions. Powder coatings typically exhibit superior chip resistance, abrasion resistance, and overall durability compared to liquid coatings. Their uniform thickness and excellent coverage make them ideal for complex geometries.
Adhesion Promotion for Organic Coatings
Proper adhesion is critical for the long-term performance of organic coatings on magnesium. Without good adhesion, coatings can delaminate, allowing corrosive agents to reach the metal surface. Surface preparation, including cleaning, degreasing, and the application of conversion coatings (like chromate-free or phosphate coatings), significantly promotes adhesion. These pretreatments create a suitable surface profile and chemical bonding sites for the organic topcoat.
Performance in Various Corrosive Environments
Organic coatings, especially powder coatings, perform well in various corrosive environments. In 90% relative humidity (RH), the formation of passivating carbonate corrosion products, such as magnesium carbonate, contributes to a longer lifetime and milder corrosion environment. This differs from constant immersion, where rapid dissolution and deterioration occur without carbonate formation.
Magnesium-rich coatings protect the underlying substrate through barrier, cathodic, and chemical protection. Cathodic protection occurs because magnesium, being more active, preferentially corrodes over the more noble substrate. This depresses the open circuit potential to more negative values. The formulation of these coatings near the critical pigment volume concentration ensures continuous electrical contact among the metal particles and with the substrate. This allows the active metal particles to act as sacrificial anodes. The formation of passivating corrosion products, such as magnesium carbonate in 90% RH conditions, also contributes to protection. It forms a protective layer that extends the coating’s lifetime. Coated magnesium exhibits a more negative potential than its bare counterpart, indicating the coating protects the magnesium substrate.
Metallic Coatings for Advanced Corrosion Resistance
Metallic coatings offer robust solutions for enhancing the durability of magnesium die casting parts. These coatings provide a physical barrier and can also offer cathodic protection, significantly extending the lifespan of components in aggressive environments.
Electroplating Techniques (Nickel, Zinc)
Electroplating involves depositing a thin layer of another metal onto the magnesium surface using an electric current. Nickel and zinc are common choices for electroplating magnesium. Nickel plating provides a hard, wear-resistant surface with good barrier properties. It can also serve as an excellent base for subsequent organic coatings. Zinc plating, on the other hand, offers sacrificial protection. Zinc is more electrochemically active than magnesium, so it corrodes preferentially, protecting the underlying magnesium substrate. However, electroplating directly onto magnesium presents challenges due to magnesium’s high reactivity and the formation of a passive oxide layer. Pre-treatment steps, such as zinc immersion or electroless nickel plating, are often necessary to ensure good adhesion and a uniform coating.
Physical Vapor Deposition (PVD) Coatings
Physical Vapor Deposition (PVD) techniques apply thin, hard films to magnesium alloys. These coatings provide both high hardness and good corrosion resistance. PVD processes involve vaporizing a source material and depositing it onto the substrate in a vacuum environment. TiMgAlN coatings, for example, deposited using both DC and HiPIMS (High Power Impulse Magnetron Sputtering) technology, successfully withstood a 48-hour salt spray test without showing corrosion attack. TiMgGdN-HiPIMS coatings demonstrate even more exceptional corrosion resistance, enduring over 360 hours in salt spray tests. Alloying with Gadolinium (Gd) enhances corrosion properties, whereas Yttrium (Y) does not provide similar benefits. The corrosion resistance of TiMgN coated magnesium alloys is significantly influenced by deposition conditions and coating microstructure. PVD coatings contribute to tribological improvement, protecting surfaces from wear and corrosion, and enhancing their appearance, thereby extending the lifespan of mechanical components.
Chemical Vapor Deposition (CVD) Coatings
Chemical Vapor Deposition (CVD) involves a chemical reaction between gaseous precursors at the substrate surface, forming a solid film. CVD coatings can produce highly pure, high-performance materials. They offer excellent adhesion and conformality, meaning they can coat complex shapes uniformly. While less common for magnesium than PVD due to the higher temperatures typically involved, specialized low-temperature CVD processes are under development. These processes aim to deposit protective layers like silicon carbide or aluminum oxide, which can significantly improve the surface properties of magnesium.
Challenges and Solutions in Metallic Coating Application
Applying metallic coatings to magnesium presents several challenges. Magnesium’s high electrochemical activity can lead to poor adhesion and galvanic corrosion if the coating is porous or damaged. The potential difference between magnesium and many common coating metals can accelerate corrosion at defects. Solutions include meticulous surface preparation, such as cleaning and activation, to ensure strong adhesion. Using intermediate layers, like zinc immersion or electroless nickel, can create a more compatible surface for subsequent metallic coatings. Furthermore, selecting coating materials that are less noble or have a smaller potential difference with magnesium can mitigate galvanic corrosion risks.
Hybrid Coating Systems for Optimized Corrosion Resistance
Hybrid coating systems combine different coating types to leverage their individual strengths, creating a multi-functional protective barrier. This approach often yields superior performance compared to single-layer coatings.
Combining Conversion Coatings with Organic Topcoats
A common hybrid strategy involves applying a conversion coating as a base layer, followed by an organic topcoat. The conversion coating, such as a phosphate or chromate-free treatment, provides initial passivation and enhances the adhesion of the subsequent organic layer. The organic topcoat, like paint or powder coating, then acts as a primary barrier against moisture and corrosive agents. This combination offers robust protection, as the conversion layer protects the magnesium even if the organic topcoat sustains minor damage.
Multi-Layered Approaches for Extreme Environments
For extreme environments, multi-layered approaches are essential. These systems often involve several distinct layers, each contributing to the overall protection. Hybrid coating systems for magnesium and its alloys often involve a Plasma Electrolytic Oxidation (PEO) base coating. This PEO coating is then impregnated with a corrosion inhibitor, such as sodium oleate (NaOl), and a polymer like polycaprolactone (PCL). Specific examples of these hybrid coatings include HC-SO 0.05–2, HC-SO 0.1–2, and AT-Mg+HC SO. Manufacturers form these coatings through sequential impregnation of PEO-coated samples in solutions of NaOl and PCL. Another method involves one-stage application of PCL and NaOl in a dichloromethane solution (HC-SO 0.05–1, HC-SO 0.1–1). The combination of sodium oleate and polycaprolactone significantly improves the corrosion behavior of PEO-treated Mg and its alloys. The HC-SO 0.1–2 (for MA8 alloy) and AT-Mg+HC SO (for AT-Mg) samples demonstrated the strongest anticorrosive properties based on EIS, PDP, hydrogen evolution, and mass-loss tests.
Synergistic Effects of Different Coating Types
The effectiveness of hybrid coating systems often stems from synergistic effects, where the combined performance exceeds the sum of individual layers. The addition of hybrid reinforcement (B4C and BN) to magnesium alloys leads to increased corrosion resistance. This synergistic effect is attributed to factors such as galvanic coupling, the formation of interfacial phases, and microstructural changes occurring between the reinforcements and the magnesium matrix. A two-step surface treatment, combining anodisation and silanisation, significantly enhances corrosion resistance and barrier properties of magnesium alloys. This combination improved these properties by three orders of magnitude compared to a blank coating or an anodised layer alone. This demonstrates a clear synergistic effect where individual layers offer limited protection but together provide substantial improvement.
Design Principles for Mitigating Corrosion in Magnesium Parts
Preventing Galvanic Corrosion Through Thoughtful Design
Material Selection for Fasteners and Inserts
Designers carefully select materials for fasteners and inserts. They choose materials compatible with magnesium. This prevents galvanic corrosion. For example, using aluminum or properly coated stainless steel fasteners reduces reactivity.
Electrical Isolation of Dissimilar Metals
Electrical isolation is crucial. Designers use non-conductive barriers, such as gaskets or washers, between magnesium and dissimilar metals. This breaks the electrical circuit. It stops the electron flow that drives galvanic corrosion.
Strategic Placement of Components
Strategic placement of components also helps. Engineers avoid direct contact between magnesium and more noble metals. They also design parts to prevent electrolyte accumulation in critical areas. This minimizes corrosion risk.
Geometric Design for Enhanced Corrosion Resistance
Eliminating Crevices and Moisture Traps
Geometric design plays a vital role. Designers eliminate crevices and moisture traps. These areas can collect water and corrosive agents. Such accumulation accelerates localized corrosion. Smooth, continuous surfaces are preferred.
Promoting Drainage and Air Circulation
Promoting drainage and air circulation is important. Designs should allow water to flow off surfaces easily. Good air circulation helps dry surfaces quickly. This reduces the time magnesium parts are exposed to moisture.
Avoiding Sharp Edges and Stress Concentrators
Designers avoid sharp edges and stress concentrators. Sharp edges can lead to localized stress. This makes the material more vulnerable to stress corrosion cracking. Rounded corners and smooth transitions improve durability.
Surface Finish and Its Impact on Corrosion Resistance
Importance of Smooth Surface Finishes
A smooth surface finish is critical. It reduces the surface area exposed to corrosive environments. Smooth surfaces also provide fewer sites for corrosion initiation. This enhances the overall durability of the part.
Effect of Surface Roughness on Coating Adhesion
Surface roughness significantly impacts coating adhesion.
- Machining, shot peening, or grit blasting can introduce interfacial defects like laps, seams, and burrs. These defects create distinct charge distribution effects. Charge concentrations occur at protrusions. Reactivity decreases at roots of discontinuities during anodizing. Such defects can lead to localized high interfacial heat, outgassing, burning, or the formation of rounded voids in the coating.
- Inclusions, intermetallic compounds, precipitates, and other insoluble alloying elements (e.g., lead, hypereutectic silicon) do not anodize. The primary oxidation reaction proceeds around them. This incorporates them into the anodic oxide. This disruption can lead to irregular growth, increased surface roughness, and reduced cohesive strength of the finish. Mass transport of these species can also occur through the coating network. This further retards oxidation kinetics.
- The overall quality of the die casting, including grain size, intermetallic formation, and phase distribution, is crucial. For instance, in high silicon alloys, silicon particles do not anodize and can lead to irregular finish thickness, excessive roughness, and reduced cohesive strength. Elements like copper and zinc, even when in solution, can cause interfacial resistance and heat, resulting in irregular, blotchy surface appearances or blisters if trapped and oxidized. The inherent chemical reactivity of magnesium leads to the formation of an oxide/hydroxide layer on its surface when exposed to air or water. This layer significantly impairs coating adhesion and uniformity. This underscores the critical importance of proper precleaning processes for achieving effective protective coatings on magnesium and its alloys.
Minimizing Surface Defects and Porosity
Minimizing surface defects and porosity is essential. High-quality die casting processes reduce these imperfections. A uniform microstructure, with controlled grain size and phase distribution, also contributes to a better surface. This ensures coatings adhere well and perform effectively.
Maintenance and Environmental Control for Sustained Corrosion Resistance
Regular Inspection and Cleaning Protocols
Importance of Routine Visual Inspections
Routine visual inspections are crucial for maintaining magnesium die casting parts. These checks allow personnel to identify early signs of degradation, such as discoloration, pitting, or coating damage. Early detection prevents minor issues from escalating into significant structural problems. Regular monitoring ensures the long-term reliability and performance of components in service.
Recommended Cleaning Agents and Methods
Manufacturers employ specific cleaning protocols to prepare magnesium surfaces and prevent corrosion. This process often begins with mechanical cleaning procedures, such as grinding, to remove surface contaminants. Chemical cleaning then prepares the surface for further treatment. Acid pickling and activation refine the surface. A typical sequence includes a detergent clean, an alkaline clean, and acid etching. Finally, a conversion coating is applied, followed by thorough rinsing and drying. These steps ensure optimal surface conditions for protective layers.
Early Detection and Remediation of Corrosion
Detecting corrosion early allows for timely intervention. Prompt remediation prevents widespread damage and costly repairs. Technicians can address localized corrosion spots before they compromise the part’s structural integrity. This proactive approach extends the service life of magnesium components.
Environmental Management to Preserve Corrosion Resistance
Minimizing Exposure to Aggressive Chemicals
Minimizing exposure to aggressive chemicals is vital for magnesium parts. Certain substances, like strong acids, alkalis, and some chlorides, accelerate degradation. Engineers design systems to shield magnesium components from direct contact with these corrosive agents. Proper material handling and storage practices also reduce chemical exposure risks.
Controlling Humidity and Temperature
Controlling environmental factors like humidity and temperature significantly impacts magnesium’s durability. High humidity levels promote moisture condensation, creating an electrolyte for corrosion. Elevated temperatures can accelerate chemical reactions. Maintaining stable, moderate humidity and temperature conditions in storage and operational environments helps preserve part integrity.
Protective Storage Conditions for Magnesium Parts
Magnesium parts require specific storage conditions to prevent degradation. Storing them in dry, climate-controlled environments minimizes exposure to moisture and temperature fluctuations. Using protective packaging, such as sealed bags with desiccants, further shields components from ambient humidity. These measures ensure parts remain in optimal condition until deployment.
Repair and Refurbishment of Corroded Magnesium Components
Techniques for Localized Corrosion Repair
Technicians can repair localized corrosion on magnesium components using several techniques. These methods often involve carefully removing the corroded material, followed by surface preparation. Mechanical abrasion or chemical etching can clean the affected area. This prepares the surface for subsequent protective treatments.
Reapplication of Protective Coatings
After localized repair, reapplying protective coatings is essential. This step restores the barrier protection lost during the corrosion process. Depending on the original coating system, technicians may apply conversion coatings, organic paints, or other specialized finishes. Proper reapplication ensures the repaired area matches the original part’s protective qualities.
Extending Service Life Through Maintenance
Comprehensive maintenance practices significantly extend the service life of magnesium components. Regular inspections, appropriate cleaning, and timely repairs prevent premature failure. Environmental control and protective storage further contribute to longevity. These combined efforts maximize the operational lifespan of magnesium die casting parts.
Achieving robust Corrosion Resistance in magnesium die casting parts presents a complex yet attainable goal. A holistic strategy integrates advanced alloy selection, sophisticated surface treatments, and meticulous design. Continuous maintenance and environmental control further ensure long-term performance and reliability. These combined efforts unlock the full potential of magnesium in diverse and demanding applications.
FAQ
What makes magnesium susceptible to corrosion?
Magnesium exhibits high electrochemical reactivity. It readily loses electrons in aqueous environments. This anodic behavior causes it to oxidize and degrade easily. This inherent tendency makes magnesium prone to various forms of corrosion.
How do metallic impurities affect magnesium’s corrosion resistance?
Metallic impurities like iron, nickel, and copper act as cathodic sites. They accelerate electrochemical reactions. This increases the corrosion rate of magnesium alloys. Manufacturers strictly control impurity levels to improve resistance.
What role does aluminum play in magnesium alloy corrosion resistance?
Aluminum significantly enhances corrosion resistance in magnesium alloys. It forms a protective alumina (Al2O3) barrier in the surface film. This barrier makes the surface more stable and resistant to corrosive attack.
What are the benefits of rare earth elements in magnesium alloys?
Rare earth elements stabilize corrosion products and refine grain structure. They also modify intermetallic phases. This leads to more uniform corrosion attack and reduced localized pitting. They improve high-temperature creep resistance.
What is Plasma Electrolytic Oxidation (PEO) / Micro-Arc Oxidation (MAO)?
PEO/MAO is an advanced anodizing process. It creates highly protective, ceramic-like coatings on magnesium alloys. This electrochemical surface treatment uses plasma discharges. It transforms the metal surface into a dense, hard oxide layer.
Why are chromate conversion coatings being phased out?
Chromate conversion coatings are being phased out due to environmental concerns. They involve toxic hexavalent chromium (Cr6) ions. Legislative restrictions, such as EU directives, also limit their use.
How does galvanic corrosion occur in magnesium parts?
Galvanic corrosion happens when magnesium contacts a more noble metal. An electrolyte must also be present. Magnesium, being more active, acts as the anode. It then corrodes preferentially.
What is the importance of surface finish for corrosion resistance?
A smooth surface finish reduces the exposed surface area. It also provides fewer sites for corrosion initiation. This enhances the overall durability of the part. Smooth surfaces also improve coating adhesion.

